A SPOTLIGHT ON THE BLIND SPOT
by Alexander Demmer
EXECUTIVE SUMMARY
- 68% of defects in industrial production are caused by humans. Even in interconnected and smart Industry 4.0 environments, human activities are a blind spot
- There is a next-gen technology entering the market: Video-based AI error recognition technology makes the production process more reliable: It supports individuals in complying with hygiene, safety, and quality requirements by spotting irregularities in real time
- In a highly regulated industry where minute errors can lead to high follow-up costs, video-AI error recognition, operating as invisible Cobots (electronical partners) prevents production delays, downtimes, recalls, recourse claims, material waste and accidents, this way significantly reducing error-related costs
- Video-based AI error recognition will considerably facilitate compliance with GMP requirements and improve margins
“Image recognition is about pixel by pixel analysis in order to recognize a particular object. A new technology is entering the market: Video-based AI Error Recognition recognizes anomalies and patterns in video data and interprets them just like a human eye and brain. This new technology has the potential to disrupt how errors on the shop floor are prevented, recognized and corrected.”
Marc Vorderman, Director Europe, Novatek International
PHARMA 4.0
There has been an escalating need for an uninterrupted supply of drugs globally with new discoveries of diseases and persisting chronic illnesses on the rise. These emerging global needs will require optimized efficiencies within each function in the pharmaceutical industry, especially within manufacturing.
Clearly, the time is right and the industry is well equipped to face the upcoming challenges. A multitude of technological innovations is currently changing the nature of manufacturing across the globe and in all sectors. The promise of Industry 4.0 in pharma – Pharma 4.0, describes a smart factory: highly automated, adaptive devices and facilities, governed by control systems that integrate, monitor and analyse all parts of the manufacturing process with sensors acting as “organs of perception”.
AUTOMATION FACILITATES GMP
Pharma manufacturing is a highly regulated, demanding environment with zero error tolerance. Minute production irregularities, non-compliance to safety and hygiene requirements can have serious consequences. In order to ensure the strength of the active ingredients as well as quality and purity of the final products, high standards need to be maintained throughout the entire production process. Flaws and irregularities can lead to substantial production losses and severe planning and logistic issues, even resulting in reputational damage.
It is obvious that increasing automation will support pharma´s constant search for perfection. Sensors measure parameters like position, velocity, pressure, vibration, temperature. They will become increasingly important to streamline and monitor the production process and provide real-time data for further processing and analysis. In this whole framework, optical sensors play an important role. These expert cameras typically analyse and verify objects that have a constant shape, texture and form, by comparing objects in a 2D setting; pixel by pixel.
HUMANS IN THE PHARMA MANUFACTURING PROCESS: A MIXED BLESSING
While Industry 4.0 has its clear focus on machines, humans still perform nearly three in four tasks on the production floor-while remaining almost invisible to analytics. Therefore, what´s missing from the automation conversation – though not from the production floor – is humans.
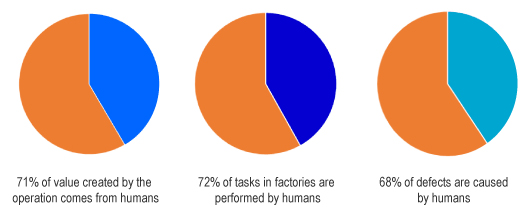
Despite their clear benefit – humans bring reason, creativity and problem-solving skills and continue to add the majority of the value in manufacturing operations. Their contribution however has one significant disadvantage: they introduce variability, which opens the door to errors and defects. According to the AT Kearney study: “The state of human factory analytics” 1, 68 percent of defects are caused by humans.
Traditionally, error mitigation strategies have been training and supervision. However, training cannot fully eliminate human errors. Supervision by humans is error-intensive, biased and insufficient. Minute safety and compliance errors can cause huge follow-up costs: material waste, production delays, recalls, reputational damage as well as health risks.
What brings human intelligence and the required scrutiny together? Cobots might be the answer to the high standards that pharma manufacturing sets forth: rather than replacing, Cobots augment the human skillset and take over the repetitive, accuracy, availability part in the equation.
THE INVISIBLE COBOT: VIDEO BASED AI ERROR RECOGNITION
According to Athina Kanioura, Chief Analytics Officer and Global Lead – Accenture Applied Intelligence in her research report: Human AI is here,
“There is tremendous potential in humans working collaboratively with machines to develop new forms of intelligence and apply them to business … problems.” 2
Human motions, gestures, characteristics are 3D, their activities are clearly too complex to be recognized, let alone traced, analysed by traditional optical sensors. It clearly needs a more sophisticated AI technology: a smart, pattern recognizing Artificial Intelligence System that understands anomalies and outliers against a constantly changing backdrop and signals non-compliance activities and threats.
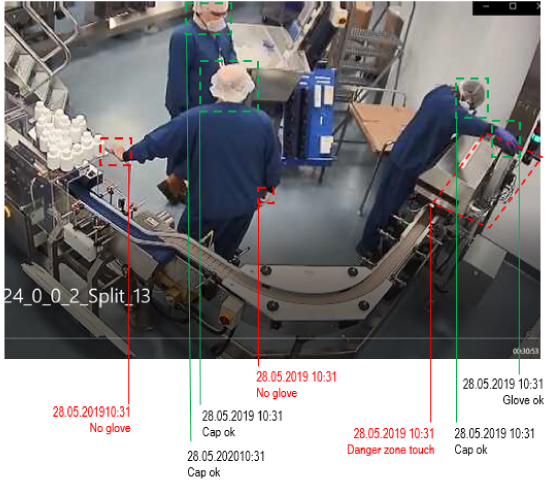
Video-based AI error recognition is a technology that operates in the background and does not require any operation training or specific skills. Standard video cameras equipped with video-based AI error recognition detect narrowly defined anomalies, e.g.:
- Safety or production risks arising from unusual or unwanted movements or gestures
- Scratches, deformations, faulty packaging, faulty assembly, number of objects, filling levels, anomalies in shape or color caused by human error
- Leaks in containers or anomalies in materials like fluids, compounds, powder that would otherwise need closer inspection by humans
Once the technology has detected an anomaly, the system triggers an alert. This can be audible, visible or an automated action like stopping the process and allowing to correct the error.
EXAMPLES: DETECT SAFETY, NON-COMPLIANCE THREATS, QUALITY ISSUES
Pharma manufacturers leverage video-based AI error correction to mitigate error-related cost:
- An operator has forgotten to put on his head cap, goggles, gown or gloves, thus violating hygiene requirements
- An operator is injured while trying to remove an obstructing object in the production line, instead of calling the service team
- A bottle on the packaging line is without a label, not correctly closed or broken
- Safety equipment like a fire extinguisher or a safety shower is blocked by an object, not a human
- Leaks in connectors to a bioreactor or other vessel are detected that would open an ingress route for contaminants
In many more situations, video-based AI error-recognition can drastically enable error correction “on the spot”. Coming back to the examples, a camera at the entrance of a hygiene area would “see” that the cap, goggles, gown or gloves are missing and an audible alarm hint at the fact that safety instructions are not observed. The technology spots the “anomaly” bottle and ejects it or halts the process, so it can be removed. It will instantly recognize that it is an object – not an individual – which obstructs free access to the life-saving equipment. A set of cameras will detect liquid on the bioreactor surface as well as spillage on the floor. Video based AI error-recognition works like a manager in the background who warns of dangers and enables quick corrections. AI error-recognition automates tedious monitoring tasks that otherwise humans would perform. In fact, the technology “sees” like a human, but does not get tired or distracted, it does not need to be organized in shifts, it does not ask for holidays or sick leave. It operates reliably 24/7.
AUTOMATING PROCESS DOCUMENTATION
The pharmaceutical industry is already experiencing regulatory scrutiny in a number of areas, including quality control activities around manufacturing operations. For example, the European Commission requires that:
“…records are made, manually and/or by recording instruments, during manufacture which demonstrate that all the steps required by the defined procedures and instructions were in fact taken and that the quantity and quality of the product were as expected. Any significant deviations are fully recorded and investigated” 3
Video-based AI error-recognition makes it easy to trace back errors during production and packaging. The solutions record and filter all deviations from the normal process and make it easy to spot the moment where the anomaly happened. Administrative staff working on investigation reports addressing complaints will never again need to crawl through many, many hours of video footage in order to find that one irregularity that spoiled that specific batch.
IMPLEMENTING VIDEO-BASED AI ERROR-RECOGNITION: STEPS TO SUCCESS

Video-based AI error-recognition offers valuable insights and reduces error-related cost. In order to make projects successful, companies however need competent partners and a clear strategy:
Business View: Video-based AI error recognition projects need a mentor not only from a technical but very much from the business perspective: Where do errors happen frequently causing severe financial impact? It is crucial to clearly identify the pain and risk and from there build the business case. Expert partners will discuss and assess in detail how video-based AI error recognition can make a difference.
Fit for Purpose Data: As AI Systems need to be trained, relevant data is crucial for the adaptation and implementation period. Expert partners will give advice on what content is relevant for reaching the defined goal and accuracy. With the right data, they will adapt the standard video-based AI Solutions to match customers´ specific requirements.
Scale: Innovation projects need a clear business perspective. What next steps are required to make the technology available to a larger audience? How can the solution be implemented across business lines, geographies and production sites? AI projects need a strategic scaling plan in order to yield business value.
Cultural Fit and Maturity to Transform: It is obvious that in a company with a strong DNA in Lean Management (a culture of Kaizen / Continual Improvement) and intensive experience with various transformational waves the success of projects of this kind will be accelerated. Technology is improving processes creating new roles and responsibilities by unburdening highly qualified employees of standard activities. The way those will be upgraded to execute more sophisticated tasks will increase the acceptance of the organization to support this kind of innovations.
Economic Value: When it comes down to cost and project duration, standard video-based AI error recognition solutions are by no means comparable with typical IT implementations or Industry 4.0 projects. Implementation and deployment projects usually take just a few months, mainly due to the learning capabilities of AI Systems. The total costs of ownership, depending on the number of potential anomalies that need to be monitored, are easy to calculate similar to the return on investment compared to expenses caused by inadequate human behaviour. ROIs below six months are not uncommon.
CONCLUSION – BUSINESS VALUE FOR PHARMACEUTICAL COMPANIES
Pharma companies continue to face challenges of globalization, complex supply chains and hyper-competition – all while demand for treatments continues to increase. As a result, the need for greater throughput, higher quality and reduced costs has become a top priority.
Video-based error-recognition for pharmaceutical development and production is a smart new technology that will replace tedious, error-intensive human supervision thus avoiding production delays, accidents, material waste, recalls, compliance fulfilment and reputation damage. In the pharma world, it will make a real difference both in competitiveness and cost reduction.

WHAT’S NEXT?
Advanced AI algorithms are screening the hidden relationships between events and behaviour building evolved models to predict results with high accuracy. High complex calculations can be executed with limited computing power with technology working on small devices.
Start-up companies are driving the race with fresh ideas and innovative test models. Among those DeepEyes is a good example of how technology can be made available to any kind of manufacturer by offering solutions working on any data base, neural networks, internet data sources as well as standalone without internet access. The algorithms operating in real-time are edge-capable, they don’t need cloud access for the analysis of video streams and are running on standard hardware and standard cameras.
The combination of IIoT (Sensors) with AI is ringing a new era in which again the humans make the difference!
1 A.T. Kearney: The State of Human Factory Analytics https://www.kearney.com/digital-transformation/the-state-of-human-factory-analytics
2 Accenture, February 2019: Human AI is here https://www.accenture.com/au-en/insights/digital/human-ai-here
3 https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/pdfs-en/2005_10_gmp_part1_chap1_en.pdf

